 |
The Meoto-Iwa, or
Wedded Rocks, Shima Peninsula. Legend holds that the spirits of
Izanagi and Izanami, Japan's creator gods, are housed in the
rocks, which are connected to one another by a straw rope.
Courtesy Corbis |
|
Clocks in Rocks:
Isotopes and Age of Earth |
In this lecture we learn:
- What is the age of the Earth and how
is it determined?
- How have theories of the age of the
Earth evolved over time?
- More on atoms: isotopes
- How are radioactive elements used to
date the age of rocks and minerals?
- When was our solar system formed
relative to the Big Bang?
Early thought
William Thompson (later Lord
Kelvin) determined the age of the Sun by calculating the time it would
take to cool to its present conditions. Later, Kelvin's calculations used
Earth's temperature change with depth, thermal properties of rocks, and a
planetary body that started as a molten mass, to produce ages in the range
of 50-100 my. This determination was firmly grounded in the physics of
late 19th Century, so its results were considered indisputable.
We will not give its derivation, but we will experiment with Kelvin's
calculation. The relationship is:
age = (To - T)2/ ( pi*K*GG2),
where To is the formation
temperature, T is today's temperature, pi is 3.14, K is a
material property called thermal diffusivity (we'll use 1mm2/sec)
and GG is the Earth's geothermal gradient (25 C/km). If To
ranges from 1500 to 2000 C, the age of the earth would range from 36-65
m.y. It was hard to argue with such sound physics, until a major discovery
was made around the turn of the century: radioactivity.
The Atom and Radiogenic Dating
Up to about silica, the number of protons in an element equals the number
of neutrons. Heavier elements can have several isotopic numbers, meaning
different numbers of neutrons, but the same number of protons. For
example, the element rubidium has the isotopes 85/37 Rb and 87/37 Rb. The
discovery of radioactivity was that the occurrence of some isotopes is
unstable, such that a new element is formed spontaneously. Of the two Rb
isotopes, 87/37 Rb is unstable and it changes to the element strontium
(87/38 Sr) by the conversion of a neutron into a proton and an electron.
The electron is expelled from the nucleus of the new element, which
produces a dangerous side effect: radiation. This type of
radioactive decay is called beta decay (b). There are several types
of radioactive decay, which are illustrated in the Figure. A useful
source of information is the
Nuclear Wall Chart.




Types of radioactive decay.
Alpha decay (a)
is the emission of particles that contain two protons and two
neutrons (He). This results in a daughter with a lower atomic
number (-2) and a lower mass number (-4). Beta decay (b)
describes the emission of an electron, which converts a neutron into
a proton. The atomic number increases by 1, whereas the mass
number remains the same. A another form of beta decay
is when a nucleus catches an electron, resulting in the conversion
of a proton to a neutron. This electron capture process,
results in a decrease in atomic number, but no change in mass
number. Gamma decay (g)
produces gamma rays, which is electromagnetic radiation from photon
emission. |
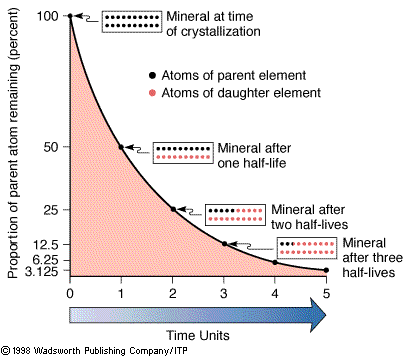
The half-lives of an element. |
In the language of radioactivity, rubidium would be called the parent
isotope and strontium the daughter isotope. The number of
isotopes that decay per unit time is proportional to the total number of
parent isotopes present. A convenient measure to express this property is
through the concept of the half-life (t½) of an isotope. The half-life is
the time required for half of a given number of parent isotopes to decay
to a daughter isotope.
The table below lists common radiogenic
systems, their corresponding half-lives and decay constants.
For example, it takes nearly 49
billion years to change 50% of Rb into Sr.
Commonly Used
Long-Lived Isotopes in Geochronology
Radioactive
Parent (P) |
Radiogenic
Daughter (D) |
Stable
Reference (S) |
Half-life, t½
(109 y) |
Decay constant,
l
(y-1) |
| 40K |
40Ar |
36Ar |
1.25 |
0.58x10-10 |
| 87Rb |
87Sr |
86Sr |
48.8 |
1.42x10-11 |
| 147Sm |
143Nd |
144Nd |
106 |
6.54x10-12 |
| 232Th |
208Pb |
204Pb |
14.01 |
4.95x10-11 |
| 235U |
207Pb |
204Pb* |
0.704 |
9.85x10-10 |
| 238U |
206Pb |
204Pb* |
4.468 |
1.55x10-10 |
Note: * 204Pb is not
stable, but has an extremely long half life of ca. 1017 years.
A useful analogy to illustrate the
fundamentals of geochronology is an hourglass. If we start with one side
of the hourglass full (containing the 'parent') and the other side empty
(containing the 'daughter'), we only need to know the rate at which the
sands moves from one chamber to the other (represented by the half-life)
and the amount of sand in the daughter chamber or the amount of parent
remaining to determine how much time has passed. However, in reality
matters are more complex.
A complication occurs in natural
samples because at the time the radiogenic clock starts ticking, the
sample already contains some daughter material; in other words, some sand
is already present in the daughter chamber even before we begin measuring
time. This amount of daughter is referred to as the initial daughter.
Therefore, when we measure the amount of daughter product in our specimen
we are combining the amounts of daughter from decay of the parent and
initial daughter. The amount of initial daughter, however, needs to be
subtracted for age determination.
The solution to this problem lies in
first determining the amount of initial daughter. The actual method is a
little tricky, but basically what we need is to find a part of the sample
that contains no radiogenic 87Rb. The measured 87Sr in that part of the
sample must therefore be initial daughter (i.e., non-radiogenic in
origin). The tricky part comes from the fact that such a component cannot
be found, but the same result may be obtained using components (minerals)
of the sample that contain different amounts of 87Rb.
Age of Earth and the Solar System
 From the age of meteorites from the asteroid belt between Mars and Jupiter, we
conclude that the solar system must be 4.56 Ga as they were formed from
the original cloud that formed the solar system. Chondrules represent the
earliest products of the solar nebula, which is supported by their
chemistry. Thus, the age of meteorites equals that of the formation of the
planets and, within a few million years, that of the formation of the Sun.
From the age of meteorites from the asteroid belt between Mars and Jupiter, we
conclude that the solar system must be 4.56 Ga as they were formed from
the original cloud that formed the solar system. Chondrules represent the
earliest products of the solar nebula, which is supported by their
chemistry. Thus, the age of meteorites equals that of the formation of the
planets and, within a few million years, that of the formation of the Sun.
Radiogenic
age measurements on rock and minerals from Earth are not that old. The
oldest rock, found in northern Canada, is about 4 Ga, whereas the oldest
mineral is about 4.3 Ga. Samples collected through the lunar program of
the late 60s and early seventies, however, support older ages. The first
moon rock picked up was dated at 3.6 billion years old! All moon rocks
examined to date are in the range 3.1 - 4.6 billion years old.
Take a trip with Berkeley's
geological time machine to learn about Earth's long and varied
history.
Summary
The age of the Earth is estimated by
using the principles of radioactive decay to date meteorites. This
technique is also applied to date rocks and minerals. The Earth is
estimated to be ~4.56 Ga and therefore formed long after the Big Bang.
Radioactive decay is the spontaneous
decay of an isotope (the parent) to a new isotope (the daughter), which is
accompanied by radiation. This process is usually described in terms of
its half-life (t½), which is the amount of time that it takes for half of
the initial parent to decay. Since half-lives can be calculated from
laboratory experiments, the only other information needed to determine the
age is the amount of parent and daughter isotopes present in the sample.
If the Universe is about 15 Ga old,
our solar system must have been formed long after the Big Bang. Supporting
evidence for this conclusion can also be found in the chemistry of our
solar system, where we find elements that cannot be formed by the fusion
process that fuels our Sun.
|
 |
Earth's Early Years:
Differentiation,
Water
and Early Atmosphere |
We learn:
-
What happened to the Earth and Moon
during the first few hundred million years?
-
How did the core and mantle form?
-
What was is the origin of the atmosphere
and ocean?
-
What is the role of early life
Differentiation: A Molten Planet
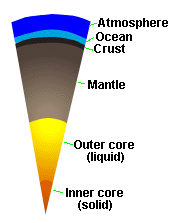 |
| Internal Structure of Earth |
Earth's solid body is composed of several layers of varying density (see
Figure). The Earth's core is composed of two portions, an inner core
of solid iron and an outer core of molten iron (perhaps with some S). Above
the core lies the mantle, which is made up of dense silicates,
and the crust, which is the outer layer of the solid Earth. The
oceans and atmosphere are the outermost layers.
Differentiation in the first few 100's of
millions of years led to the formation of the core and the mantle and a crust,
and
initiated the escape of gases from the moving interior that eventually
led to the formation of the atmosphere and oceans.
Heating of Early Earth
The earliest Earth was probably an unsorted
conglomeration, mostly of silicon compounds, iron and magnesium oxides,
and smaller amounts of all the natural elements. It became increasingly
hotter as the protoplanet grew. Four different effects led to the heating
of our planet:
1. Accretion. Impacting bodies
bombard the Earth and convert their energy of motion (kinetic energy) into
heat. In recent years we also learned that an early collision with a very
large object was responsible for the "extraction" of the Moon from Earth.
2. Self-compression. As the
Earth gets bigger, the extra gravity forces the mass to contract into a
smaller volume, producing heat (just like a bicycle pump gets hot on compression).
3. Differentiation. Conversion
of gravitational potential energy to heat during core formation
3. Short-lived radiogenic isotopes.
The surrounding material absorbs the energy released in radioactivity,
heating up. Today this is a very slow but steady source of heat. About
20 calories of heat are generated by 1 cubic centimeter of granite in the
course of a million years. It would take this amount of rock 500 million
years to brew a cup of coffee!
Iron Melts
At some point, probably within the
first few hundred million years of Earth, the surface down to a depth of
about 500 km became so hot that iron (a plentiful element) started to melt.
The molten iron collected and began to sink under its own great weight.
About one third of the primitive planet's material sank to the center,
and in the upheaval, heating rates increased and most of the planet was
liquified. There might well have been an early ocean of molten rock -- a magma
ocean more than 100 km deep. The formation of a molten iron core
was the first stage of the differentiation of the Earth, in which it was
converted from a homogenous body, with roughly the same kind of material
at all depths, to a layered body, with a dense iron core, a crust composed
of lighter materials with relatively lower melting points, and between
them the mantle.
 |
The melting
of iron leads to the
formation of a
heavy liquid layer.
Drops begin to develop
in later stages and
sink toward the center. |
The Figure below compares the elemental
abundances for the Earth's crust with the whole Earth, showing that
the crust has a quite different composition from the rest of the Earth,
with abundant oxygen and silicon. About 90% of the
Earth is made of the four elements iron, oxygen, silicon and magnesium.
|
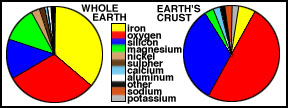
Comparison of relative abundances of
elements in (a) the Earth's crust and
(b) the whole Earth. |
Compare the abundance of elements in
the crust with the values for the Earth as a whole. Because most of the
iron sank to the core, that element drops to fourth place. Conversely,
silicon, aluminum, calcium, potassium, and sodium are far more abundant
in the crust than in the whole Earth. The reason for the different make
up is that the elements favored in the crust form light-weight chemical
compounds, which are easily melted. Materials such as these melted early
during the differentiation, rose to the surface by convective overturning
and accumulated.
The Earliest Atmosphere, Oceans and
Continents
After loss of the hydrogen, helium and
other hydrogen-containing gases from early Earth due to the Sun's radiation,
primitive Earth was devoid of an atmosphere. The first
atmosphere was formed by outgassing of gases trapped in the interior of
the early Earth, which still goes on today in volcanoes.
For the Early Earth, extreme volcanism occurred during differentiation, when massive heating
and fluid-like motion in the mantle occurred. It is likely that the bulk
of the atmosphere was derived from degassing early in the Earth's history.
The gases emitted by volcanoes today
are in Table 1 and in Figure.
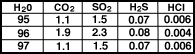 |
Composition of volcanic
gases for three volcanoes |
|
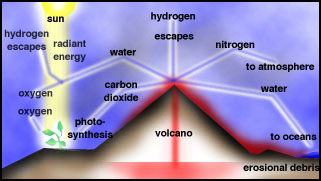 |
Volcanic outgassing |
Oxygen in the Atmosphere

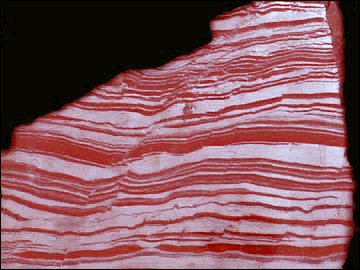
Stromatolite and Banded-iron Formation (BIF)
Life started to have a major impact on the environment once
photosynthetic organisms evolved. These organisms, blue-green algae (picture of
stromatolite, which is the rock formed by these algae), fed off atmospheric
carbon dioxide and converted much of it into marine sediments consisting
of the shells of sea creatures.
While photosynthetic life reduced the carbon dioxide content of the
atmosphere, it also started to produce oxygen. For a long time, the oxygen
produced did not build up in the atmosphere, since it was taken up by rocks, as
recorded in Banded Iron Formations (BIFs; picture) and continental red beds. To this day, the majority of oxygen
produced over time is locked up in the ancient "banded rock" and "red
bed" formations. It was not until probably only 1 billion years ago that
the reservoirs of oxidizable rock became saturated and the free oxygen
stayed in the air.
Once oxygen had been produced, ultraviolet light split the
molecules,
producing the
ozone UV shield as a by-product. Only at this point
did life
move out of the oceans and respiration evolved. We will discuss these
issues in greater detail later on in this course.
Early Oceans
The Early atmosphere was probably dominated
at first by water vapor, which, as the temperature dropped, would
rain out and form the oceans. This would have been a deluge of truly global
proportions an resulted in further reduction of CO2. Then the atmosphere
was dominated by nitrogen, but there was certainly no oxygen
in the early atmosphere. The dominance of Banded-Iron Formations (BIFs; see
picture)
before 2.5Ga indicates that Fe occurred in its reduced state (Fe2+). Whereas
reduced Fe is much more soluble than oxidized Fe (Fe3+), it rapidly oxidizes
during transport. However, the dissolved O in early oceans reacted with
Fe to form Fe-oxide in BIFs. As soon as sufficient O entered the atmosphere,
Fe takes the oxidized state and is no longer soluble. The first occurrence
of redbeds, a sediments that contains oxidized iron, marks this major transition
in Earth's atmosphere.
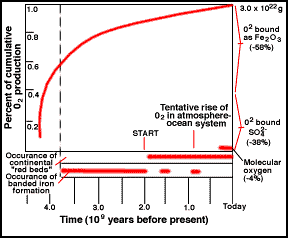 |
Cumulative history of O2 by photosynthesis over geologic time.
The start of free O is likely earlier than shown. |
Early Continents
Lava flowing from
the partially molten interior spread over the surface and solidified to
form a thin crust. This crust would have melted and solidified repeatedly,
with the lighter compounds moving to the surface. This is called differentiation.
Weathering by rainfall
broke up and altered the rocks. The end result
of these processes was a continental land mass, which would have grown over
time. The most popular theory limits the growth of continents to the first
two billion years of the Earth.
Additional Work
Run through and complete
Virtual Dating - Isochron to learn more about isotopic dating.
All materials © the
Regents of the University of Michigan unless noted otherwise.















