
Ozone:
The Good, The Bad and The Ugly
"There are strange things done in the midnight sun...".
Robert Service, "The Cremation of Sam McGee""
Suggested Readings:
- Scientific American Readings, "Managing Planet Earth", W. H. Freeman, New York, 1990.
- James C. White., "Global Climate Change Linkages", Elsevier, 1989.
- Richard P. Wayne, "Chemistry of Atmospheres", Oxford, 1991.
- Jonathan Weiner. "The Next One Hundred Years", New Sciences, Bantam, 1991.
- Sayed Z. El-Sayed, "Fragile Life under the Ozone Hole", Natural History, October 1988, pp 73-80.
We wish to learn:
- What chemistry controls the ozone layer?
- How might the ozone layer react to anthropogenic changes?
- How does society react to alarming scientific findings?
| Introduction
To Global Change I - Lecture Notes |
|
|||
| Ozone as a Greenhouse Gas | Stratospheric Ozone | Tropospheric Ozone |
Summary | |
1. Ozone as a Greenhouse Gas
 |
| Figure 1. Relative importance of the five most important greenhouse gases. |
In the past twenty years, it has become increasingly evident that certain trace gases play a major role in determining the climate system - far in excess of what might be thought based on their small numbers. Carbon Dioxide is perhaps the principal culprit for potential global warming, but it is by no means the only one.
Figure 1 shows the relative contribution to tropospheric warming due to the greenhouse effect of various gases. This plot, taken from model calculations, contains two surprises. Firstly, the Chloro-fluorocarbons (CFCs) taken as a whole (there are several members of this family of gases) represent the second most important gas for global warming - even though their concentrations are measured in the parts per trillion, as opposed to parts per billion for carbon dioxide and methane. The CFCs are entirely of anthropogenic origin.
Secondly, we see that both ozone and nitrous oxide (N2O or "laughing gas") are significant greenhouse gases. In fact, most gases that are made up of three or more atoms are effective greenhouse gases. This is because they have the ability to absorb and emit infra-red radiation via processes of rotational and vibrational excitation (think, for example, of the three atoms making up CO2 as being connected by springs - infra red light is emitted and absorbed in association with the jiggling and spinning of the springed molecule).
For a full study of the issues relating to Global Change, therefore, we need to account quantitatively for the sources and sinks of all these greenhouse gases, incorporating a discussion of the extent to which their presence in the atmosphere can be attributed to human activities and a projection of their future abundances.
Tables 1 and 2 provide more detailed summaries of some of the attributes of important trace gases that are found in the Earth's atmosphere. Table 1 lists the major anthropogenic sources for each trace gas, as well as the mean residence time and the projected change in abundance with time. The last column of Table 1 provides an estimate for the projected concentration of the gas in the year 2030 in parts per billion (ppb), based on a conservative assumption for future global industrial development.
Table 2 provides information on the two principal concerns we always have when discussing a trace gas, namely:
- what is its the "Greenhouse Potential" (GP)?
- what is its the Ozone Depletion Potential (ODP)?
For convenience, both GP and ODP are measured on a per molecule basis, using as reference the potentials of specific CFC molecules. Thus, for example, we see from Table 2 that a molecule of methane has only 0.001 times the effectiveness of a molecule of CFC-12 for greenhouse warming. Similarly, we see that Carbon Dioxide is not a particularly effective greenhouse gas on a per molecule basis (GP = 0.00005), but since it is much more abundant than the others, it still comes out on top (see Figure 1).
|
|
|
TOTAL EMISSIONS PER YEAR (MILLIONS OF TONS) |
|
|
|
|
|
|
|
|
|
40-80, S. Hem. (Clean Atmospheres) |
40-80, S. Hem. (Clean Atmospheres) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Clean to Industrial) |
(Clean to Industrial) |
(Clean to Industrial) |
|
|
|
|
|
|
|
|
|
|
|
|
|
(Clean to Industrial) |
(Clean to Industrial) |
(Clean to Industrial) |
|
|
|
|
|
|
(Chlorine atoms) |
(Chlorine atoms) |
|
|
|||||
|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
There are many other things to note about the data of these tables. Notice, for example, the long atmospheric lifetimes of some of the gases - 60-100 years for CFCs, 170 years for N2O, etc. Clearly, climate changes induced by anthropogenic effects via these gases will take a long time to undo.
Also, all the trace gases of Tables 1 and 2 have significant anthropogenic (human) sources. In many cases, it is via fossil fuel burning - but cattle raising, rice production, deforestation, fertilizer application, ore smelting, motor vehicle emissions, aerosol sprays, etc., also all play a role. The production of trace gases is seen to be part and parcel of our industrial and agricultural civilization - it will not be politically easy to make great changes. In fact, the data of Table 1 predict continuing accumulation of these gases in the atmosphere.
Notice also that it is the chlorine- or bromine-bearing (CFC-family-member) gases that have the greatest ozone-destroying potential - more on that below.
Resources: |
2. Stratospheric Ozone (The Good News)
From the above discussion, we can see that ozone not only protects us from UV light - it is also a greenhouse gas in its own right. We next focus on chemistry of ozone - how is it produced and how is it destroyed?
Stratospheric Ozone Abundance
 |
|
| Figure 2. Ozone concentration as a function of height above the South Pole on 16 October, 2001. Click image to investigate other days in 2001. | |
Figure 2 is a reminder of the measured abundance of ozone in the stratosphere. Ozone occurs in a layer, centered at around 30 km altitude, reaching a peak abundance of ~10 parts per million. Even at the peak of the ozone layer, however, it is still very much a trace constituent - two orders of magnitude down from CO2 and 5 or 6 orders down from O2 and N2. If we were to take all the ozone in a column overhead and bring it down to sea level (room temperature and pressure) it would occupy a layer of only 3 mm in thickness!
It is interesting to notice how different the ozone distribution is from most of the other gases shown in Figure 2. Ozone occurs in a layer, while the other gases have simple exponential drop-offs with altitude (straight lines on this logarithmic plot).
Why does stratospheric ozone exist is a layer? To answer this question, we need to understand the production mechanism for ozone.
Ozone Production
Ozone is a deep blue, explosive, and poisonous gas. It is made in the atmosphere by the action of sunlight on molecular oxygen. In the stratosphere, UV light is available that can split up ordinary molecular oxygen into two atomic oxygen atoms.
O2 + UV photon --> O + O
Now, atomic oxygen is a very reactive species - so much so that it is very hard to make in the laboratory - it immediately combines with something else. In the stratosphere, atomic oxygen can quickly combine with molecular oxygen (in the presence of a third body) to yield the almost equally reactive other allotrope of oxygen: ozone or O3.
O + O2 + third body --> O3 + third body
The combination of these two reactions, mediated by sunlight, converts molecular oxygen into ozone. Thus ozone is continually being created in the stratosphere by the combination of molecular oxygen and sunlight.
Ozone Layering
 |
| Figure 3. Production of the Ozone Layer in the Stratosphere. The two ingredients for stratospheric ozone production are molecular oxygen and UV sunlight. |
We can now explain why ozone is created in a layer in the stratosphere. Figure 3 illustrates the altitude dependence of the ozone production rate.
On the topside of the layer, production is limited by the availability of molecular oxygen (which is dropping off exponentially with altitude as shown in Figure 2).
On the bottom side of the layer, production is limited by the availability of UV sunlight (which gets rapidly absorbed by ozone itself).
The net effect of these two factors is to produce the characteristic layer for ozone.
Ozone Loss
Ozone is lost through the following pair of reactions:
O3 + UV photon --> O2 + O
O + O3 --> 2O2
The first of these two reactions serves to regenerate atomic oxygen for the second reaction which converts the ozone back to molecular oxygen. This second reaction is very slow. It can be enormously accelerated, however, by catalytic reactions (see below). In the absence of such catalytic reactions, ozone can survive for 1-10 years in the stratosphere.
Catalytic Destruction of Ozone by Chorine from CFCs
 |
|
Figure 4. Current ozone column depth over southern
hemisphere |
Catalysis refers to the acceleration of a particular chemical reaction by a catalyst, a substance that is not destroyed in the reaction, enabling it to continue having the same accelerating effect time and time again.
Rapid catalytic destruction of ozone is best explained by reference to the famous example of CFCs (also known as freons) in the stratosphere.
Chlorofluorocarbons (CFCs) were developed to be colorless, odorless, non-staining, chemically inert, non-toxic, non-flammable, and to have certain other properties that make them excellent refrigerants, solvents, propellants for aerosol cans, and foam-blowing agents. These same properties make them essentially inert in the troposphere.
In the stratosphere, however, the CFCs can be broken apart into more reactive fragments under the action of UV light. When this splitting occurs, free chlorine is liberated which can catalytically destroy ozone. The process occurs in two steps:
Step 1
"Photolysis" (splitting by sunlight) of CFCs in the stratosphere
Cl2CF2 + UV light --> ClCF2 + Cl
Step 2
Catalytic destruction of ozone
Cl + O3 --> ClO + O2
ClO + O3 --> Cl + 2O2
Notice that the net effect of this pair of fast reactions is to turn two ozone molecules into three normal molecules of oxygen. The (catalyst) atomic chlorine is recovered in the second reaction, making it available to start over. In fact, each chlorine atom can destroy hundreds of thousands of ozone molecules!
These two steps turn a very unreactive chemical into a devastatingly effective destroyer of ozone. Whenever free chlorine atoms exist in the stratosphere, ozone is quickly depleted. Other species (such as bromine and fluorine) can also act as ozone-destroying catalysts.
Given this chemistry, it is useful to consider a typical life history of CFCs in the atmosphere:
- Spray starch aerosol can is emptied in Ann Arbor
- The CFC is rapidly dispersed until it is uniformly distributed throughout the troposphere. It takes about a year to mix across into the southern hemisphere as well, carried by weather patterns
- After a few years, some of the CFC leaks into the stratosphere. At a sufficiently high altitude (~30 km), the available UV light can photolyze the CFC, liberating chlorine.
- Each atom of chlorine participates in the catalytic destruction of thousands of molecules of ozone.
- Eventually the chlorine atom reacts with methane to produce HCl, a molecule of hydrochloric acid.
- Some of the HCl reacts with OH to liberate Cl again, but a small fraction of it mixes down into the troposphere where it can dissolve in rainwater and be lost to the atmosphere through precipitation.
- The time scale for this process is ~100 years!
Potential Effects of Depleted Ozone
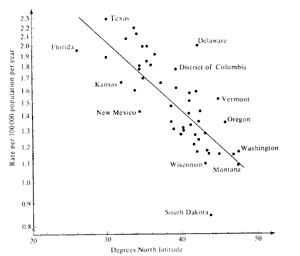 |
| Figure 5. Rate of change of skin cancers versus latitude. |
Of primary concern are the enhanced levels of UV radiation that reach the Earth's surface for a depletion in stratospheric ozone. It is customary to break up the UV spectrum into two parts:
UV-A: 400 - 320 nm
UV-B: 320 - 290 nm
The more energetic UV-B portion of the spectrum is responsible for sunburn, cataracts, potential ecological damage, and skin cancer. It can be absorbed by glass as well as by sunscreens and hats.
Relatively little is known or understood about the consequences of enhanced UV-B levels. We do know, however, that a 1% decrease in ozone abundance causes a ~2% increase in UV-B. Table 3 summarizes some of the potential effects of UV-B increases.
|
|
||||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
|
|
|
|
||||
|
|
|
|
||||
|
|
|
|
||||
|
|
|
|
||||
|
|
|
|
||||
|
|
|
|
||||
|
|
|
| ||||
|
||||||
Our best understanding of potential effects is in the area of skin cancers, for which detailed epidemiological records and studies exist. It is known, for example, that more than 90% of non-melanoma skin cancers are related to UV-B exposure. A 2% increase in UV-B is linked with a 2-5% increase in basal-cell cancer cases and a 4-10% increase in squamous-cell cancer cases.
In 1990, there were ~500,000 cases of basal-cell cancer in the U.S. and ~100,000 cases of squamous-cell cancer. A 1% depletion of ozone would cause an increase in skin cancer cases of ~20,000 per year.
To put this rather alarming figure in context, it is necessary to discuss briefly the geographical prevalence of skin cancer in the U.S. Figure 5 illustrates the rate of skin cancer as a function of latitude. While the data has some scatter, the trend is clear. A decrease of ~110 in latitude results in an increase of a factor of 2 in skin cancer occurrence. This occurs because the UV-B exposure increases towards the equator (~ a factor of 50 from pole to equator).
An increase of ozone of 1% gives an increase of ~20,000 cases of skin cancer per year. This is equivalent to a southward shift in the average latitude of the U.S. population by only ~12 miles. Actual ozone depletions at the latitude of the U.S. are ~1-3% already - primarily caused by chlorine catalytic chemistry.
 |
| Figure 6. The size of the 2000 Southern Hemispohere ozone hole (Click on image for enlargement). [Source: NOAA] |
Figure 6 illustrates the size of the Antarctic ozone hole for this season as estimated from satellite data. Notice the changes in relative size from previous years.
It is thought that ozone levels have been already depleted globally due to the CFC emissions and consequent chlorine catalysis.
The depletions occur at all latitudes and seasons, but are most dramatic in the southern polar region in austral springtime (October). This depletion is the famous Antarctic Ozone Hole.
Changes in Ozone
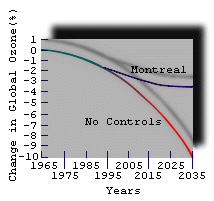 |
| Figure 7. Percentage change in global ozone predictions with and without compliance with the Montreal Protocol, an international agreement which phases out CFC production by 2000. |
Theoretical models have been developed to predict future changes in ozone abundance. Figure 7 shows the results of one such projection into the future.
The Montreal Protocol was signed in 1987 and has since been strengthened. It commits to phase out production of the CFCs (first invented in 1930) by the turn of the century.
Without the Montreal Protocol, we would be looking at a disastrous reduction in ozone levels.
3. Ozone in the Troposphere (The Bad)
Primary NAAQS: the levels of air quality that the EPA judges necessary, with an adequate margin of safety, to protect the public health. Secondary NAAQS: the levels of air quality that the EPA judges necessary to protect the public welfare from any known or anticipated adverse effects. |
When fossil fuels (e.g., gasoline) are burned, a variety of pollutants are emitted into the earth's troposphere, i.e., the region of the atmosphere in which we live - from ground level up to about 15 km. Two of the pollutants that are emitted are hydrocarbons (e.g., unburned fuel) and nitric oxide (NO). When these pollutants build up to sufficiently high levels, a chain reaction occurs from their interaction with sunlight in which the NO is converted to nitrogen dioxide (NO2). NO2 is a brown gas and at sufficiently high levels can contribute to urban haze. However, a more serious problem is that NO2 can absorb sunlight and break apart to produce oxygen atoms that combine with the O2 in the air to produce ozone (O3).
Ozone is a powerful oxidizing agent, and a toxic gas. In North America elevated levels of tropospheric ozone cause several billion dollars per year damage to crops, structures, forests, and human health. It is believed that the natural level of ozone in the clean troposphere is 10 to 15 parts-per-billion (ppb). Because of increasing concentrations of hydrocarbons and NO in the atmosphere, scientists have found that ozone levels in "clean air" are now approximately 30 ppb. A principal activity of atmospheric chemists is to study and determine how we might reverse this trend.
Photochemical smog is one of the most important and obvious air pollutants. An important role in the air pollution chemistry, especially in the formation of ozone is played by nitrogen oxides, NOx which stands for a group of compounds including nitric oxide (NO) and nitrogen dioxide (NO2). These compounds, along with other hazardous gases, are emitted when coal is burned in power plants and industrial boilers for the generation of power, and from automobiles. Most of the NOx emitted from combustion is nitric oxide, formed according to the following reaction.
N2 + O2 = 2NO
The high temperatures (3000°F to 4000°F) which are maintained in the combustion favor the formation of NO.
The following reactions can also take place in the furnace, in the stack,
or later, in the atmosphere:
NO + O2 = 2NO2
NO2 + NO = N2O3
2NO2 = N2O4
3NO2 + H2O = 2HNO3 + NO
Nitrogen dioxide (NO2) reacts with hydrocarbons which are
present in the atmosphere to form aldehydes and ketones through photochemical
reactions. It also can react with oxygen in the presence of sunlight to
give nitric oxide and ozone:
NO2 + O2 = NO + O3
Ozone is a pollutent when breathed and the U.S.E.P.A. has designated a National Ambient Air Quality Standard (NAAQS) for ozone (see table to right) that its concentration should not exceed 125 ppb over a one hour period.
4. Summary
- Various greenhouse gases are accumulating in the atmosphere due to
human activities, these include CFCs, N2O, methane and CO2.
- Some of these gases also affect stratospheric ozone concentrations.
Ozone depletions occur when catalytic chemistry accelerates natural
loss processes. An important catalytic loss reaction chain involves
chlorine, made available in the stratosphere by the photolysis of CFCs.
- Ozone reductions (estimated to be ~1-3% globally) can cause environmental problems due to the increase in UV-B radiation. One such problem involves increases in skin cancer. A recovery to natural ozone levels would take ~100 years in the complete absence of further CFC emissions.